Weight Loss Plan Biography
Source:- Google.com.pkAs a dietary supplement, take one or two capsules in the morning and one or two capsules if needed in the afternoon with water. Do not take within 5 hours before bed time. Consider taking this product in combination with BioSynergy BioEndurance, L-carnitine, and Hoodia.
*ChromeMate® is a registered trademark of InterHealth Nutraceuticals.
Caution: Keep out of the reach of children. Not intended for persons under 18 years of age. Do not use if you are pregnant or nursing or at risk of or being treated for high blood pressure, heart disease, renal disease, hyperthyroidism, spasms, psychiatric disease or suffer from migraines.
Bio-G™ Benefits:
Green Tea Extract
If diet and exercise aren’t helping you shed pounds fast enough, green tea extract may give you the boost you’re after, suggests Chris D’Adamo, Ph.D., an assistant professor at the University of Maryland’s center for integrative medicine. A 2009 analysis of 11 studies showed that the combination of caffeine and epigallo catechin gallate (EGCG) found in green tea led to a small but significant weight loss.
Life is more rewarding when you are mentally and physically fit.
Safety of Bitter Orange Documented in Scientific Review
A review published in Phytotherapy Research provides an in depth scientific analysis of the chemistry and safety of bitter orange drawn from 89 clinical research and other reference sources. The article concludes that the “preponderance of human clinical studies have reported that bitter orange extract (synephrine) either alone or in combination with caffeine and other ingredients has no effect on blood pressure or heart rate.”
After critically reviewing the available published chemical, pharmacological, toxicological and clinical data available on bitter orange and its extracts, the authors of the HerbalGram safety review concluded that there is confusion in the medical literature that has caused misinformation among health professionals, journalists and others:
“In summary,” they wrote, “based on current research as well as the extensive ingestion of bitter orange and p-synephrine in the form of dietary supplements as well as fruits, juices and other citrus food products, the data demonstrates that bitter orange extract is safe for human consumption. No credible adverse events have been directly attributed to bitter orange or its primary protoalkoloid, p-synephrine, in association with oral ingestion.”
“Due to the widespread confusion about the relative safety of bitter orange, we employed an extra level of peer review for this article,” said Mark Blumenthal, founder and executive director of ABC and editor of HerbalGram. “We normally use three expert peer reviewers for articles in HerbalGram,” he noted, “but for this article we went an extra few miles by having additional expert reviewers comment on and check the accuracy of all of the information in this safety review.”.
Developmental Toxicity of Citrus aurantium in Rats
Deborah K. Hansen, Division of Personalized Nutrition and Medicine, NCTR/FDA, Jefferson, Arkansas
Abstract
BACKGROUND: Ephedra was commonly used in herbal products marketed for weight loss until safety concerns forced its removal from products. Even before the ban, manufacturers had begun to replace ephedra with other compounds, including Citrus aurantium, or bitter orange. The major component in the bitter orange extract is synephrine which is chemically similar to ephedrine. The purpose of this study was to determine if relatively pure synephrine or synephrine present as a constituent of a bitter orange extract produced developmental toxicity in rats.
METHOD: Sprague-Dawley rats were dosed daily by gavage with one of several different doses of synephrine from one of two different extracts. Caffeine was added to some doses. Animals were sacrificed on GD 21, and fetuses were examined for the presence of various developmental toxic endpoints.
RESULTS AND CONCLUSION: At doses up to 100 mg synephrine/kg body weight, there were no adverse effects on embryolethality, fetal weight, or incidences of gross, visceral, or skeletal abnormalities. There was a decrease in maternal weight at 50 mg synephrine/kg body weight when given as the 6% synephrine extract with 25 mg caffeine/kg body weight; there was also a decrease in maternal weight in the caffeine only group. This decrease in body weight may have been due to decreased food consumption which was also observed in these two groups. Overall, doses of up to 100 mg synephrine/kg body weight did not produce developmental toxicity in Sprague-Dawley rats. Birth Defects Res (Part B) XX:1–8, 2011. © 2011 Wiley-Liss, Inc.
Bio-G™ Side Effects:
At this time there are no clinically proven side effects with Bio-G™ supplement when used as directed . If you are taking any prescribed drugs from your physician, please check the drug interactions before taking this nutritional supplement.
Caution: Keep out of the reach of children. Not intended for persons under 18 years of age. Do not use if you are pregnant or nursing or at risk of or being treated for high blood pressure, heart disease, renal disease, hyperthyroidism, spasms, psychiatric disease or suffer from migraines.
Drug Interactions
Bio-G™ Dosage:
Suggested Usage: As a dietary supplement, take one or two capsules in the morning and one or two capsules if needed in the afternoon with water. Do not take within 5 hours before bed time. Consider taking this product in combination with BioSynergy BioEndurance, L-carnitine, and Hoodia.
Bio-G™ is a great aid to your weight loss/diet & natural energy supplement program.
Scroll up to add Bio-G™ to your cart!
*The above statements have not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.
Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram
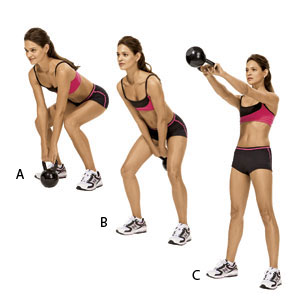
Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

Weight Loss Plan Weight Loss Tips Tumblr For Women In Urdu By Dr Khurram In Urdu By Zubaida Tariq Urdu In Urdu For Girls And Tricks In Urdu By Doctor Khurram

No comments:
Post a Comment